- Research
- Open access
- Published:
Needs-based triggers for timely referral to palliative care for older adults severely affected by noncancer conditions: a systematic review and narrative synthesis
BMC Palliative Care volume 22, Article number: 20 (2023)
Abstract
Background
Older people with noncancer conditions are less likely to be referred to palliative care services due to the inherent uncertain disease trajectory and a lack of standardised referral criteria. For older adults with noncancer conditions where prognostic estimation is unpredictable, needs-based criteria are likely more suitable. Eligibility criteria for participation in clinical trials on palliative care could inform a needs-based criteria. This review aimed to identify and synthesise eligibility criteria for trials in palliative care to construct a needs-based set of triggers for timely referral to palliative care for older adults severely affected by noncancer conditions.
Methods
A systematic narrative review of published trials of palliative care service level interventions for older adults with noncancer conditions. Electronic databases Medline, Embase, CINAHL, PsycINFO, CENTRAL, and ClinicalTrials.gov. were searched from inception to June 2022. We included all types of randomised controlled trials. We selected trials that reported eligibility criteria for palliative care involvement for older adults with noncancer conditions, where > 50% of the population was aged ≥ 65 years. The methodological quality of the included studies was assessed using a revised Cochrane risk-of-bias tool for randomized trials. Descriptive analysis and narrative synthesis provided descriptions of the patterns and appraised the applicability of included trial eligibility criteria to identify patients likely to benefit from receiving palliative care.
Results
27 randomised controlled trials met eligibility out of 9,584 papers. We identified six major domains of trial eligibility criteria in three categories, needs-based, time-based and medical history-based criteria. Needs-based criteria were composed of symptoms, functional status, and quality of life criteria. The major trial eligibility criteria were diagnostic criteria (n = 26, 96%), followed by medical history-based criteria (n = 15, 56%), and physical and psychological symptom criteria (n = 14, 52%).
Conclusion
For older adults severely affected by noncancer conditions, decisions about providing palliative care should be based on the present needs related to symptoms, functional status, and quality of life. Further research is needed to examine how the needs-based triggers can be operationalized as referral criteria in clinical settings and develop international consensus on referral criteria for older adults with noncancer conditions.
Background
Inequities in the provision of palliative care remain globally, whilst palliative care should be available to all who need it regardless of their diagnosis [1]. Global ageing and the changes in the prevalence of diseases imply that most needing palliative care worldwide are older people living with noncancer conditions [1,2,3,4]. However, there is consistent evidence that older people with noncancer conditions experience inequitable access to palliative care with low rates of referral or late referral in the last days or weeks of life [5, 6], such as in dementia [7] and heart failure [8].
There are several major barriers to referral in patients with noncancer conditions. A systematic review reported that one of the barriers to access and referral to palliative care is ‘a lack of national standardised referral criteria’ for screening patients with chronic noncancer disease regarding their need for palliative care [9]. A questionnaire survey showed that the highest barrier perceived by specialist palliative care service providers was ‘the unpredictable noncancer disease trajectory’ [10]. In primary care settings, the uncertainty of the illness trajectory was also identified as a barrier to effective primary palliative care provision for noncancer patients [11]. As a result, in clinical practice, key triggers or prompts for older adults with noncancer conditions to access palliative care are based on variable professional opinions or experiences [12, 13]. This means referral triggers are typically informed by differences in education, interest, and understanding on the intended outcomes of palliative care.
Referral criteria are needed to address the inequity of access to palliative care for older adults with noncancer conditions. Systematic review on referral criteria for noncancer patients aged over 65 years identified predictor variables to aid clinicians’ prognostic estimation [5]. However, the inherent uncertain disease trajectory for older adults with noncancer conditions requires the provision of palliative care to be based upon need, rather than time-based criteria, such as disease trajectory and prognostic criteria [13, 14]. Although systematic reviews identified referral criteria for palliative care among patients with heart failure [15, 16], dementia [17], and Parkinson’s disease [18], there has been no referral criteria based on palliative care needs for older general noncancer populations and the reviews assert the lack of consensus on palliative care referral criteria for adults with noncancer conditions.
Eligibility criteria for participation in clinical trials on palliative care can inform a needs-based criteria for palliative care. This approach was used and advocated by Hui et al. [12] and others [15, 17, 18] in systematic reviews investigating eligibility criteria for trials to inform triggers for outpatient palliative cancer care. Hui et al.’s review identified six themes for referral, two time-based, including cancer trajectory and prognosis, and four needs-based, including physical symptoms, performance status, psychosocial distress, and end-of-life care planning [12]. As appropriate trial eligibility criteria are designed to measure efficacy of an intervention, the criteria include populations considered likely to benefit from the intervention compared with the control. Eligibility criteria in palliative care trials seeks to identify patients with palliative care needs and considered likely to benefit from palliative care. Although eligibility criteria for randomised controlled trials (RCTs) may not be specifically designed for referral, they can inform a needs-based set of triggers for timely referral to palliative care.
Aim
This systematic review aimed to identify, appraise the applicability, and synthesise patient eligibility criteria in published trials on palliative care service level interventions for older adults severely affected by noncancer conditions. The findings intended to construct a needs-based set of triggers for timely referral to palliative care.
Methods
Study design
A systematic narrative review of the published literature on palliative care interventions for older adults with noncancer conditions to identify and synthesise the criteria used to indicate eligibility for palliative care provision [19]. The review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2020 guidelines [20]. The PRISMA 2020 checklist is shown in Additional file 1. The methodological quality of the included studies was assessed using a revised Cochrane risk of bias tool for randomized trials [21]. The protocol was registered in PROSPERO in November 2018 (CRD42018095845, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018095845845). We were not required to seek an institutional ethics approval because we only used publicly accessible documents.
Data sources and searches
Relevant articles were identified from six electronic searches: MEDLINE (Ovid), EMBASE (Ovid), CINAHL (EBSCOhost), PsycINFO (Ovid), the Cochrane Central Register of Controlled Trials (CENTRAL), and ClinicalTrials.gov. Search strategies were informed by previous systematic reviews related to palliative care and older adults [12, 13, 22]. A full search strategy can be seen in Additional file 2. All searches were conducted from database inception to September 2018 and updated on June 2022. We supplemented the electronic searches with reference chaining and citation tracking, and handsearching two palliative medicine textbooks [23, 24] and conference abstracts [Research Congress of the European Association for Palliative Care (EAPC), 2018]. All identified studies were managed in EndNote. There was no language restriction in the selection of studies.
Eligibility criteria
Types of studies
We included RCTs, including cluster randomised trials, pilot, and feasibility trials. We sought to identify and collate trial eligibility criteria for patient participants and appraise what patterns of eligibility criteria were successful in terms of recruitment, attrition, attaining sample size, and effect on the primary outcome. Feasibility and pilot trials were included, as intention is to evaluate if they can recruit patients to the trial using the stated eligibility criteria. We excluded trials that focused exclusively on the economic evaluation of palliative care as not evaluating the effect of palliative care on patient outcomes, and non-experimental studies (observational studies) as our interest was patient eligibility criteria for palliative care intervention trials. We excluded opinion pieces including editorials, commentaries, letters, and dissertations.
Types of participants
We included adults (aged ≥ 65 years) severely affected by chronic noncancer illness, including chronic heart failure (CHF), chronic obstructive pulmonary disease (COPD), chronic kidney failure, cirrhosis of the liver, stroke and long-term neurological conditions, including dementia and Parkinson’s disease. These conditions can cause considerable distressing symptoms and concerns [25]. Receipt of palliative care is shown to relive suffering [26, 27]. We included studies where > 50% of the population was aged ≥ 65 years and > 50% of the population were people with noncancer conditions.
Types of interventions
We included palliative care trials to identify individuals who could benefit from palliative care. Specialist and general palliative care interventions were included that aimed to promote quality of life for adults aged older adults ≥ 65 years severely affected by noncancer conditions. We defined a palliative care intervention as a model or service of palliative care, not a discrete aspect of palliative care, such as oxygen therapy. We defined a palliative care model or service as comprising four key elements [28,29,30,31], namely:
All levels of palliative care in any setting
We referred to the model of a three-level structure: palliative care approach in all settings, general basic palliative care, and specialist palliative care with adequate skills for each level [32]. All study settings were included: community health services, including clinics and health centres, outpatient and ambulatory care settings, and inpatient units.
An intervention providing direct palliative care to older adults
Interventions that did not directly deliver care to patients were excluded (e.g., interventions to caregivers, education programs to healthcare professionals, or evaluations of assessment tools). We considered a palliative care service intervention if the authors described it as 'palliative' anywhere in the manuscript.
An intervention had multi-component services
A palliative care service is a multidimensional and holistic approach to meet the physical, psychological, social, and spiritual needs of patients. Interventions that delivered only one component of palliative care (e.g., medication, psychotherapy, complementary therapy, decision aid) were not considered as palliative care service.
An intervention was provided by a multidisciplinary team
We defined 'palliative care services' as multidisciplinary services providing comprehensive care aiming at different physical and psychosocial components of palliative care. We excluded interventions provided by only one professional (e.g., nursing intervention).
Outcome
Types of outcome measures were not restricted.
Study selection and data extraction
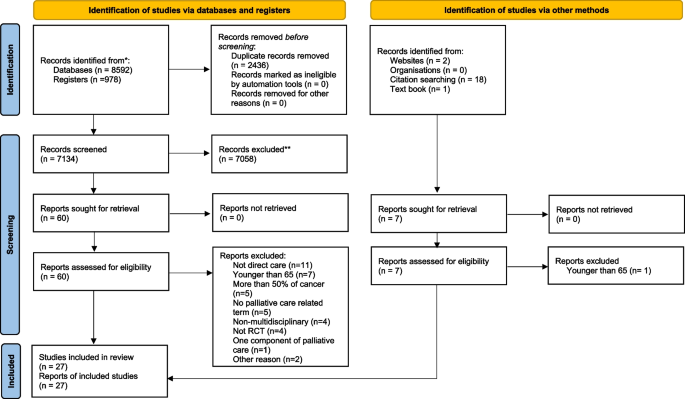
The review author A.K. screened and assessed the identified titles and abstracts according to the inclusion criteria, followed by assessing all relevant full-articles by A.K. and E.A.D.P., independently. For the update search, A.K. and R.T. assessed full-articles, independently. Disagreements were resolved by consensus and discussed with C.J.E. The inter-rater reliability between the first author A.K. and E.A.D.P. and between A.K. and R.T. were assessed with a percentage of agreement. The selection process was presented in a PRISMA 2020 flow diagram (Fig. 1) [20]. Data were extracted by A.K. The PRISMA guideline [20] informed the data extraction detailing trial eligibility criteria, target population, impact on clinical outcome, and stated limitations, study design, study aim, including intervention, participant eligibility criteria, participant characteristics, screening to recruitment rate and main outcomes.
Quality appraisal of included studies
The author A.K. assessed the risk of bias in all included RCTs, as described in the Cochrane Handbook for Systematic Reviews of Interventions [33]. A revised Cochrane risk-of-bias tool for randomized trials is composed of the following five domains of bias: risk of bias arising from the randomization process; risk of bias due to deviations from intended interventions; risk of bias due to missing outcome data; risk of bias in measurement of the outcome; and bias in selection of the reported result. The summary judgements of the level of risk of bias for each domain are presented in Table 1. We used the robvis which is a visualization tool for tabulating a table of risk of bias and categorised them as, ‘low risk’, ‘high risk’, ‘some concerns’, or ‘no information’ [34].
Data analysis and synthesis
Descriptive analysis and narrative synthesis provided descriptions of the patterns and appraised the applicability of included trial eligibility criteria to identify patients likely to benefit from receiving palliative care. We sought to identify and collate eligibility criteria and appraise what patterns of eligibility criteria were successful in terms of recruitment, attrition, attaining sample size, and effect on the primary outcome. Eligibility criteria were summarized by frequency counts of domains and synthesized by intended sample size, attrition rate, causes of attrition, and limitation that reflected on the eligibility.
To assess the recruitment, we appraised whether: 1) the study recruited the relevant population to answer the study aim, 2) the actual sample size was larger than the intended sample size determined by sample size calculation, and 3) the explanation for revision of the target sample size was given.
We assessed the attrition rate in accordance with the implementation of sample size estimation, cause of attrition, and anticipated attrition rate. Rates of attrition in the included trials were assessed to explore levels of attrition and if high attrition, to consider the appropriateness of the trial eligibility criteria to identify patients for palliative care (or not). To describe causes of attrition, we used the MORECare classification of attrition to describe causes of attrition: attrition due to death (ADD), attrition due to illness (ADI), and attrition at random (AaR) [62, 63]. While there is no standardised level of loss to follow-up which attrition related bias was identified as a problem, Schulz and Grimes noted that the readers should be concerned about the possibility of bias when the attrition rate was 20% or greater [64]. However, the weighted average attrition across palliative care trials involving adults with serious illness and increasing nearness to end of life in a systematic review was 29% [63]. For example, a review of interventional palliative oncology trials stated that the attrition rate was 26% for the primary endpoint and 44% for the end of the study [65]. Therefore, we considered high attrition rate when the rate was more than 25%. Although dropouts due to symptom progression or death were not considered as protocol failures in palliative care trials, we sought to appraise what patterns of eligibility criteria were successful in terms of recruitment and the effect on the primary outcome.
We identified for each trial the level of statistically difference on the primary outcome between the intervention and control groups and the effect size. We then explored the respective eligibility criteria and target population to map and identify criteria associated with effect on the primary patient outcome. This intended to explore further the appropriateness (or not) of the patient eligibility criteria used. Because the timing of outcome measurement could influence the attrition rate and the effect on the primary outcome, we also considered the impact of length of intervention and time points of data collection.
Results
Study selection
The electronic search strategy identified total 9,584 papers (6,720 in 2018 and 2,850 in the update search). An additional 21 papers were identified by hand searching and citation tracking. After removing duplicates, 7,134 studies were screened at title and abstract, and 67 were assessed as full-text articles. 27 met eligibility (20 studies identified by electronic searches and seven from hand search) (see Fig. 1) [35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61]. All included studies were written in English. The reasons for study exclusion are reported in Fig. 1 [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103]. The inter-rater agreement between independent reviewers of full text screening were 89% in the initial search and 86% for the update search.
Quality appraisal
Only five studies were considered low risks of bias [36, 37, 50, 52, 53]. 20 studies had high risks of bias, mainly due to incomplete outcome data. Two study were assessed at some concerns because of unstated data and lack of information [46, 56]. The risk of bias plots are presented in Additional files 3 and 4. The average attrition rate was 23%, ranging from 0 to 52%. The major cause of attrition was death. Eleven papers stated that small sample size was one of the limitations. What we valued more than attrition rate when assessing incomplete outcome data was advance estimations of attrition, descriptions of reasons for missing data, and whether they integrated these into sample size calculation.
Study characteristics
This review included 27 RCTs written in English. Most studies were conducted in US. This is important to understand the context of the work and the applicability of the proposed triggers for which settings. The first study was conducted in US in 2000 [35]. (Table 1).
Study design
We included 18 phase III RCTs and nine feasibility trials. The 15 parallel RCTs compared the palliative care intervention with usual care. Five studies used fast-track design and compared a fast-track group with a waiting list group. Higginson et al. [57] used a parallel group fast-track trial design. Finally, five studies used a mixed methods trial design [49, 50, 53, 54, 57].
Participants
The studies included 3,663 participants ranging from 14 to 517 per study [54, 55]. The mean age ranged from 65.5 years with heart failure [36] to 85.7 years with chronic noncancer conditions and frailty [53]. The female percentage ranged from 9.1% [41] to 81.8% [35]. Eleven studies described the ethnicity of the participants; the majority of participants were White, followed by African Americans.
Of the included 27 papers, eight were conducted with patients with heart failure (HF) [36, 43,44,45, 47, 50, 60, 61]. Six included participants with respiratory disease, two with COPD [58, 59], two with interstitial lung disease (ILD) [37, 49], one with idiopathic pulmonary fibrosis (IPF) [41], and one with COPD/chronic obstructive airway disease (COAD) [54]. Other diagnoses were neurological diseases, two with Parkinson’s disease (PD) [42, 52], one with dementia [35], and one stated long-term neurological conditions. Four studies [38, 48, 51, 57] included multiple diseases, three included both cancer and noncancer conditions [38, 51, 57], and one included both CHF and COPD [48]. Three studies stated general chronic/advanced noncancer populations [46, 53, 55], and one included intensive care unit (ICU) populations [40].
Intervention and control
Eleven different models of home palliative care in 14 studies were identified [48,49,50,51,52,53,54,55,56,57,58,59,60,61]. All eleven models were composed of multi-components of palliative care intervention, such as symptom management, self-management education of disease, end-of-life discussions, case conferences, documentation, regular home visits, or a telephone hotline. Seven studies [35, 38, 40, 43,44,45, 47] provided inpatient care and six [36, 37, 39, 41, 42, 46] implemented outpatient palliative care services. Inpatient palliative care services in seven studies were developed based on the standard referral process of the hospital palliative care team or developed for the trial. As for control group, usual care differed across studies due to the wide variety of health systems and local service provisions. Several studies followed national or government guidelines.
Primary outcomes
20 studies [36,37,38, 41, 42, 44,45,46,47,48,49,50, 52,53,54,55,56,57, 59, 60] set quality of life (QOL) or symptom burden as a primary outcome, with marked heterogeneity in the measurements used. (Table 1). Eight used disease-specific QOL measurements, two used HF-specific QOL measures; The Kansas City Cardiomyopathy Questionnaire (KCCQ), four used respiratory diseases specific measures; a QOL domain in the Chronic Respiratory Disease Questionnaire (CRQ), the St. George’s Respiratory Questionnaire (SGRQ), or Maugeri Respiratory Questionnaire (MRQr), and two used measures for neurological conditions; the 39-Item Parkinson's Disease Questionnaire (PDQ-39) or the Quality of Life in Alzheimer’s Disease (QoL-AD). Other primary outcomes were medical service use, documentation of care preferences, and patient satisfaction.
Summary of eligibility criteria of included studies
Six main domains for eligibility criteria were identified, including diagnostic criteria (n = 26 out of 27 included studies, 96%), medical history-based (n = 15, 56%), symptoms (physical and psychological) (n = 14, 52%), prognostic criteria (n = 9, 33%), functional status (n = 8, 30%]), QOL (n = 2 [7%]), and other criteria (n = 4, 15%). We categorised these domains into three major criteria themes: needs-based, time-based, and medical history-based.
In Table 2, we used the initial letter of each domain to show which category the eligibility trials were categorised. The letter D stands for ‘Diagnostic Criteria’, P for ‘Prognostic’, S for ‘Symptoms’, Q for ‘QOL’, F for ‘Functional Status’, M for ‘Medical History and Treatment’, and O for ‘Other’. The number of domains was calculated by adding the number of domains used in the trial as eligibility criteria. Since some standardised measures covered several domains, we analysed the domain in the measurements and counted the number of domains. For example, Bekelman et al. [36] used a score of HF-specific health status (KCCQ) to assess eligibility. As KCCQ is a reliable and valid measure of symptoms, functional status, and QOL, the number of domains counted was three [104]. Table 3 gives an overview of the different criteria and use by respective disease groups. Table 4 gives a systematization of the eligibility criteria which shows major categories for referral criteria.
Need-based criteria
Focused on three main areas of symptoms, function, and quality of life, including:
Symptoms
Around half of the 27 included studies set an existence of physical/psychological symptoms as eligibility criteria [36, 42, 45, 46, 48, 50, 53,54,55,56,57, 59,60,61]. As for physical symptoms, Aiken et al. [48] included HF or COPD patients suffering from fatigue, palpitation, dyspnoea, or angina with any activity. For participants with HF, Brännström et al. [50] checked the presence of cardiac cachexia with involuntary non-oedematous weight loss ≥ 6% of total body weight within the preceding 6–12 months, and Bekelman et al. [36] confirmed reporting at least one of the target symptoms of fatigue, shortness of breath, pain, and/or depression. Only six studies [36, 42, 53, 56, 60, 61] contained psychological symptoms as eligibility criteria. Four studies that provided breathlessness intervention/support service examined whether breathlessness existed in spite of optimisation of the underlying illness [46, 50, 54, 57]. Among them, Higginson et al. [57] used the Medical Research Council (MRC) dyspnoea scale to assess the degree of refractory breathlessness. Four studies [42, 53, 56, 59] conducted comprehensive screening of complex symptoms in palliative population.
Functional status
Eight studies included criteria that assess functional or performance status in their eligibility [35, 36, 40, 42, 46, 51, 53, 59]. In trials of patients with HF or COPD, the Palliative Performance Scale (PPS) and KCCQ were employed to assess functional status alongside prognosis, symptoms, or QOL [36, 51]. Regarding neurological conditions, Ahronheim et al. [35] used the Functional Assessment Staging Test (FAST) for systematic examination of the functional changes occurring in patients with dementia. Helgeson et al. [40] considered admission of patients with dementia from nursing care facilities as a pre-existing functional dependency. The trial eligibility criteria of Kluger et al. [42] on Parkinson's disease contain the Palliative Care Needs Assessment Tool (PC-NAT) and their criteria were based on a broad range of potential palliative care needs rather than time-based criteria.
Evans et al. [53] assessed the existence of frailty with the clinical frailty scale score. Shunk et al. [46] set the capability to participate in physiotherapy as a functional criterion because of the nature of the intervention programme.
Quality of life
Only two trials used QOL for trial eligibility [36, 50]. Bekelman et al. [36] used KCCQ in their trial as a measurement of the patient’s perception of their health status which includes how their heart failure impacts their QOL within a 2-week recall period. Brännström et al. [50] measured QOL using a Visual Analogue Scale (VAS). VAS is commonly used to rate subjective experiences [105].
Time-based criteria
Diagnostic criteria
Diagnostic criteria were a set of signs and tests for use in routine clinical care to guide the care of individual patients. In the 12 studies that included HF, six studies [43, 44, 48, 50, 60, 61] used the New York Heart Association (NYHA) classification of II-IV [43, 44], or NYHA III-IV [47, 48, 50, 60]. Brumley et al. [51] included not only participants with HF, but also COPD and cancer, and used the Palliative Performance Scale to assess disease severity. Rogers et al. [45] measured signs of volume overload in accordance with the HF diagnosis. But, three HF studies [38, 47, 57] used no diagnostic criteria.
Similarly, diagnostic eligibility criteria were used in studies on lung disease and neurological conditions. Aiken et al. [48] in a study on COPD used measures of hypoxemia, oxygen saturation, pO2, and oxygen requirements. Three studies [37, 41, 49] on ILD or IPF used high-resolution computed tomography of lung or a composite physiologic index. Janssen et al. (2019) [58] and Scheerens et al. [59] used the Global Initiative for Chronic Obstructive Lung Disease (GOLD) system to categorize airflow limitation into stages of COPD.
Regarding neurological conditions, Gao et al. [56] employed the Hoehn and Yahr scale and the Expanded Disability Status Scale (EDSS) to describe the progression of each neurological disease. Hanson et al. [39] used the Global Deterioration Scale (GDS) to assess the severity of dementia. The other seven studies did not clearly state the measurements of diagnostic eligibility criteria [40, 46, 51, 53,54,55, 57].
Prognostic criteria
Nine studies included prognostic eligibility criteria [37, 38, 40, 45, 48, 50, 51, 60, 61]. The ‘surprise question’ was used in four studies [38, 51, 60, 61]. The question is, “Would I be surprised if this patient died in the next 12 months?”, which has been used to identify patients at a high risk of death who might benefit from palliative care services [106]. Three [45, 60, 61] used HF-specific standardised prognostic measures. Ng et al. [60] and Wong et al. [61] and used multi-components of the prognostic indicator guidance to identify end-stage heart failure (ESHF) [107]. The indicators are constituted by three steps, which initiate intuitive surprise questions, followed by general and specific clinical indicators. In the three steps, they used only the last step, heart disease-specific clinical indicators. Rogers et al. [45] used the North American Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) risk score which uses clinical information to derive a discharge model for six months risk of rehospitalisation and mortality [108]. Bassi et al. [37] used the gender-age-physiology (GAP) index to estimate prognosis and enrol participants with advanced ILD [109]. Helgeson et al. [40] used APACHE and SOFA scoring models to measure severity of critically ill patients admitted to ICU and to predict their mortality [110].
Medical history-based criteria
We identified 15 articles that included medical history and treatment criteria. Among 15, eleven trials included criteria related to repeated unplanned hospital admissions due to the deterioration of illness [35, 39, 40, 43,44,45, 50, 58,59,60,61]. The study of dementia by Ahronheim et al. [35] used medical history-based eligibility criteria for hospitalization for acute illness occurred with advanced dementia. However, as many participants died when they were admitted to a hospital, the authors assert these criteria limited study recruitment and attainment of sample size. Treatment-based criteria included previous/current administrative data, such as intravenous therapy support (e.g., diuretics), intubation or non-invasive ventilation, required diuretic dosing, required long-term oxygen therapy, post-cardiac arrest, and results of heart function [36, 48, 51]. Evans et al. [53] considered increasing health service use as a concern caused by severely affected non-malignant chronic conditions.
Other criteria
We found four other criteria which can be considered as psychosocial eligibility and needs perceived by healthcare professionals. Higginson et al. [57] asked patients their willingness to engage with a breathlessness support service. Farquhar et al. [54, 55] assessed whether patients might benefit from a self-management programme. Although Helgeson et al. [40] considered the medical ICU perceived need, they did not clearly state the measurements of the criteria.
Discussion
Main findings of the study
We systematically reviewed and narratively synthesised the eligibility criteria of 27 RCTs in palliative care. The findings of our review inform development of needs-based triggers for timely referral to palliative care for older adults severely affected by noncancer conditions. The results showed the list of potential needs-based triggers which were composed of three criteria; symptoms, functional status, and QOL. Eligibility criteria that were ‘successful’ tended to utilize at least one domain of needs-based criteria. Six studies used successful eligibility criteria according to the recruitment, attrition rate, and effect on primary outcomes in each study [50, 51, 53, 54, 57, 61].
What this study adds
Decisions about informing palliative care should be based on individual needs related to symptoms, functional status, and QOL. Few studies used standardized measurements with specific cut-offs for symptoms, functional decline, or QOL assessment.
Symptoms
Comprehensive assessment aligned to philosophy of palliative care is essential to identify patients likely to benefit from receiving palliative care. In this review, trial eligibility criteria are limited to mainly physical symptoms with little consideration of psychological symptoms e.g. anxiety, depression. Outcome measures of specific physical symptoms such as pain are well developed, but psychosocial symptoms are liable to be considered less serious than physical symptoms. Furthermore, only one study used standardised measures to assess symptom control: MRC dyspnoea scale [57]. Four studies [54, 55, 60, 61] measured physical symptoms such as breathlessness in spite of optimisation of underlying illness, though the measurements are not standardised tools. It implies that using a validated measure for palliative care referral is uncommon.
The best practice of symptom assessment is patient self-report outcomes for example using patient-reported outcome measures (PROMs/PROs) rather than clinician assessment due to the subjective nature of symptoms [111]. However, considering the illness trajectory and deterioration in physical/cognitive abilities in palliative care populations and the potential burden of completing PROMs, reporting by proxies such as relatives or healthcare professionals is important, especially for older adults [112,113,114]. For older adults with noncancer conditions, loss of mental capacity is common with advancing age associated with for example severe dementia and nearness to end of life. Therefore, measures used in palliative care need to be validated for the population and clinical practice, and for both self and proxy reporting [115]. Some outcome measures include a proxy version, for example the Integrated Palliative Care Outcome Scale (IPOS) [116]. This allows for the adjusting of proxy ratings if the patient is not able to complete the measure as their disease progresses.
Functional status and quality of life
Functional status and QOL are important needs-based triggers for older adults with noncancer conditions. Functional status is defined as the level of ability to do “activities performed by an individual to realize the needs of daily living in many aspects of life including physical, psychological, spiritual, intellectual, and roles [117]. Three of the included trials indicate that using standardised measures to assess functional status of patients is important to identify individuals likely to benefit from palliative care [35, 36, 51]. Most older adults severely affected by noncancer conditions experience progressive functional disability and subsequent health decline during the course of their disease. Moreover, some studies reported that functional status is significantly associated with health-related QOL (HRQOL) in people with noncancer conditions [118, 119]. Although disease-specific functional assessment measures can be available in some noncancer diseases, the Australia-modified Karnofsky Performance Status (AKPS) is a modified version for palliative care that is widely used and appropriate for multiple care settings in palliative care populations [120]. Collecting and evaluating data on functional status during routine care could inform the need for palliative care for timely referral.
The primary goal of palliative care for older people is to improve QOL with provision based on their needs [13, 29]. Quality of life can be defined as a complex, multifaceted construct that requires multiple approaches from different theoretical angles [121]. Although physical and psychological symptoms and functional impairment can be related to decline in QOL, QOL can be a trigger for referral as it can reflect an unmet need. In this review, eligibility criteria related to QOL were uncommon. As QOL that has a broad multidimensional concept can be difficult to be used as a single referral criterion, it could be operationalized as referral criteria in conjunction with other needs-based criteria. There are few relevant assessment tools addressing functional status and HRQOL for populations with multiple chronic conditions [122].
Willingness to engage with intervention
Psychosocial eligibility criteria were uncommon, mostly limited to views on willingness to engage with the intervention [54, 55, 57]. One of the major differences between palliative care and other fields of healthcare is the holistic approach it takes, including psychosocial and spiritual dimensions in addition to physical suffering. Willingness to receive palliative care may reflect the patient's preference and could form a needs-based trigger for a referral on preference for palliative care. However, patients who have preferences for palliative care may differ in characteristics compared to those with reluctance to refer to palliative care. For example, low level of health literacy of illness may preclude understanding on benefit of receiving palliative care service and impede individuals’ access to palliative care services. The Health Literacy Skills conceptual framework introduced by Squires et al. [123] illustrates mediators between health literacy and health outcomes. According to the framework, lack of knowledge about available palliative care services means patients do not request or access these services [124, 125]. Educating individuals about the role and function of palliative care, and confirming the willingness to engage with the intervention, may be one of the simplest ways to assess needs-based triggers for a referral on preference for palliative care.
Limited availability of validated assessment tools
A barrier to using individual needs-based triggers for referral criteria is the limited availability of validated and brief standardised assessment tools encompassing symptom severity, functional status, and QOL for older people with noncancer conditions. Whilst generic measures are able to be used on a large range of health and in various health conditions and populations, specific measures specifically developed to measure outcomes in palliative care are more responsive to needs-based triggers than generic outcome measures. As palliative care focuses on providing holistic care, the outcome measure used to assess palliative care needs for people with noncancer diseases should be comprehensive and encompass multiple health domains [126, 127]. Validated comprehensive measures for palliative care are available and used in clinical care, for example Edmonton Symptom Assessment Scale (ESAS) [128], and the IPOS [116] with condition specific measure for dementia and multi-morbidity (IPOS-Dem) [129]. They encompass holistic domains including, physical, psychological, social and spiritual dimensions.
Implications for further research and practice
Our review produced the initial step toward developing standardized referral criteria for clinical practice for older adults severely affected by noncancer conditions. Although the results inform a needs-based set of triggers for timely referral to palliative care, further research is needed to examine the feasibility, outcome and processes to operationalise the needs-based triggers as referral criteria in clinical settings.
Future research is needed to develop an international consensus on referral criteria for older adults with noncancer conditions and investigate if the developed referral criteria can be used in clinical settings to identify patients likely to benefit from receiving palliative care. The provision of palliative care should be based on needs assessment [32]. Our findings indicate that needs-based criteria are more likely to suit older people with noncancer conditions. Suitable needs-based referral criteria should meet the varied needs of people with different illness trajectories and different complexities of need for palliative care [32]. We recommend using measurements that encompass symptoms, QOL and functional status. Simple comprehensive measures developed and validated for palliative care population are practical for quick assessment of the palliative care needs, for example IPOS [116] or ESAS [128]. As palliative care needs vary widely, continued assessment of needs-based triggers are advocated. Standardised measures that can aid clinicians to assess palliative needs and concerns should be easy to use and interpret for all healthcare professionals and short to accommodate time constraints in clinical settings [130].
Strengths and limitations of the study
A key strength of this review is the identification and analysis of trial eligibility criteria for noncancer conditions without restricting by diagnosis. This intended to identify referral criteria applicable across noncancer conditions and multimorbidity. However, most participants in the included studies had heart failure or chronic respiratory disease. This strengthens the applicability of the identified needs-based set of triggers for these population groups, but may limit wider application to all older patients with other noncancer conditions. There is great heterogeneity among older people aged over 65 driven by for example variable diagnosis and multimorbidity, compared to any other age group. The impact this of heterogeneity on the recommendations for palliative care referral should be carefully considered. Though some studies that included mixed diagnosis attempted to reduce the heterogeneity of multimorbidity by identifying disease combinations, future research should consider how to manage heterogeneity, including stratification by age, diagnostic group, and number of co-morbidities. In the study selection process, although we assessed all relevant full-articles by two reviewers independently, the titles and abstracts of studies retrieved in bibliographic searches were assessed by one reviewer. Single screening of the titles and abstracts can influence the number of studies missed. Finally, the included studies were predominantly conducted in high-income countries in Europe and the US. This limits generalisability to non-Western regions and low-middle income countries.
Conclusion
The findings of this systematic review and narrative synthesis inform development of needs-based triggers for timely referral to palliative care for older people severely affected by noncancer conditions. For older people severely affected by noncancer conditions, decisions about providing palliative care should be based on the present needs related to symptoms, functional status, and quality of life. Further research is needed to examine the feasibility, outcome and processes to operationalise the needs-based triggers as referral criteria in clinical settings and develop international consensus on referral criteria for older adults with noncancer conditions.
Availability of data and materials
The PRISMA 2020 checklist, the full search strategy, and the risk of bias plots have been presented in Additional files. Any further data analysed during this study are available from the corresponding author on reasonable request.
Abbreviations
- AKPS:
-
The Australia-modified Karnofsky Performance Status
- APACHE:
-
The Acute Physiology and Chronic Health Evaluation
- CHF:
-
Chronic Heart Failure
- COAD:
-
Chronic Obstructive Airways Disease
- COPD:
-
Chronic Obstructive Pulmonary Disease
- CRD:
-
Centre for Reviews and Dissemination
- CRQ:
-
The Chronic Respiratory Disease Questionnaire
- EAPC:
-
European Association for Palliative Care
- EDSS:
-
Emergency Department
- ESAS:
-
Edmonton Symptom Assessment System
- ESCAPE risk score:
-
The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness risk score
- ESHF:
-
End-Stage Heart Failure
- FAST:
-
Functional Assessment Staging Tool
- GAP:
-
The Gender-Age-Physiology index
- GDS:
-
The Global Deterioration Scale
- GOLD:
-
The Global Initiative for Chronic Obstructive Lung Disease
- HF:
-
Heart Failure
- HRQOL:
-
Health-related quality of life
- ICU:
-
Intensive Care Unit
- ILD:
-
Interstitial Lung Disease
- IPF:
-
Idiopathic pulmonary fibrosis
- IPOS:
-
The Integrated Palliative care Outcome Scale
- KCCQ:
-
Kansas City Cardiomyopathy Questionnaire Short Version
- MRC:
-
Medical Research Council
- MRQr:
-
Maugeri Respiratory Questionnaire
- NYHA:
-
New York Heart Association
- PD:
-
Parkinson’s Disease
- PC-NAT:
-
The Palliative Care Needs Assessment Tool
- PDQ:
-
The Parkinson's Disease Questionnaire
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-analyses
- PROMs:
-
Patient Reported Outcome Measures
- PROSPERO:
-
The International Prospective Register of Systematic Reviews
- QoL-AD:
-
The Quality of Life in Alzheimer’s Disease
- RCT:
-
Randomised Controlled Trial
- SGRQ:
-
The St. George’s Respiratory Questionnaire
- SOFA:
-
The Sequential Organ Failure Assessment
- VAS:
-
Visual Analogue Scale
References
Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–90.
Hall S, Petkova H, Tsouros AD, Costantini M and Higginson IJ. Palliative care for older people: better practices. WHO Regional Office for Europe. 2011. [https://www.euro.who.int/__data/assets/pdf_file/0017/143153/e95052.pdf. ] Accessed 24 Apr 2022.
Addington-Hall J, Higginson IJ. Palliative care for non-cancer patients. Oxford: Oxford University Press; 2001.
Sleeman KE, de Brito M, Etkind S, Nkhoma K, Guo P, Higginson IJ, Gomes B, Harding R. The escalating global burden of serious health-related suffering: projections to 2060 by world regions, age groups, and health conditions. Lancet Glob Health. 2019;7(7):e883–92.
Coventry PA, Grande GE, Richards DA, Todd CJ. Prediction of appropriate timing of palliative care for older adults with non-malignant life-threatening disease: a systematic review. Age Ageing. 2005;34(3):218–27.
Burt J, Raine R. The effect of age on referral to and use of specialist palliative care services in adult cancer patients: a systematic review. Age Ageing. 2006;35(5):469–76.
Sampson EL, Candy B, Davis S, Gola AB, Harrington J, King M, Kupeli N, Leavey G, Moore K, Nazareth I, et al. Living and dying with advanced dementia: a prospective cohort study of symptoms, service use and care at the end of life. Palliat Med. 2018;32(3):668–81.
Kavalieratos D, Kamal AH, Abernethy AP, Biddle AK, Carey TS, Dev S, Reeve BB, Weinberger M. Comparing unmet needs between community-based palliative care patients with heart failure and patients with cancer. J Palliat Med. 2014;17(4):475–81.
Ahmed N, Bestall JC, Ahmedzai SH, Payne SA, Clark D, Noble B. Systematic review of the problems and issues of accessing specialist palliative care by patients, carers and health and social care professionals. Palliat Med. 2004;18(6):525–42.
O’Leary N, Tiernan E. Survey of specialist palliative care services for noncancer patients in Ireland and perceived barriers. Palliat Med. 2008;22(1):77–83.
Oishi A, Murtagh FE. The challenges of uncertainty and interprofessional collaboration in palliative care for non-cancer patients in the community: a systematic review of views from patients, carers and health-care professionals. Palliat Med. 2014;28(9):1081–98.
Hui D, Meng YC, Bruera S, Geng Y, Hutchins R, Mori M, Strasser F, Bruera E. Referral criteria for outpatient palliative cancer care: a systematic review. Oncologist. 2016;21(7):895–901.
Evans CJ, Ison L, Ellis-Smith C, Nicholson C, Costa A, Oluyase AO, Namisango E, Bone AE, Brighton LJ, Yi D, et al. Service delivery models to maximize quality of life for older people at the end of life: a rapid review. Milbank Q. 2019;97(1):113–75.
Connor SR. Global Atlas of Palliative Care, 2nd Ed 2020. Worldwide Palliative Care Alliance (WPCA). 2020. [https://cdn.who.int/media/docs/default-source/integrated-health-services-(ihs)/csy/palliative-care/whpca_global_atlas_p5_digital_final.pdf?sfvrsn=1b54423a_3.] Accessed 28 Oct 2022.
Chang YK, Kaplan H, Geng Y, Mo L, Philip J, Collins A, Allen LA, McClung JA, Denvir MA, Hui D. Referral criteria to palliative care for patients with heart failure: a systematic review. Circ Heart Fail. 2020;13(9): e006881.
Chang YK, Allen LA, McClung JA, Denvir MA, Philip J, Mori M, Perez-Cruz P, Cheng S-Y, Collins A, Hui D. Criteria for referral of patients with advanced heart failure for specialized palliative care. J Am Coll Cardiol. 2022;80(4):332–44.
Mo L, Geng Y, Chang YK, Philip J, Collins A, Hui D. Referral criteria to specialist palliative care for patients with dementia: a systematic review. J Am Geriatr Soc. 2021;69(6):1659–69.
Chen Y, Hou L, Li W, Wang Q, Zhou W, Yang H: Referral criteria to palliative care for patients with Parkinson’s disease: a systematic review. Curr Med Res Opin. 2022:1–36.
Pope C, Mays N, Popay J. Synthesizing qualitative and quantitative health evidence: a guide to methods. Maidenhead and New York: Open University Press, McGraw Hill Education; 2007.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71.
Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge SM, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366: l4898.
Gomes B, Calanzani N, Curiale V, McCrone P, Higginson IJ. Effectiveness and cost-effectiveness of home palliative care services for adults with advanced illness and their caregivers. Cochrane Database Syst Rev. 2013;2013(6):CD007760.
Bruera E Higginson IJ, von Gunten CF, Morita T: Textbook of Palliative Medicine and Supportive Care. 3rd edition.: CRC Press; 2021.
Cherny NI, Fallon MT, Kaasa S, Portenoy RK, Currow DC. Oxford Textbook of Palliative Medicine. 6th ed. Oxford: Oxford University Press; 2021.
Chaudhry SI, Murphy TE, Gahbauer E, Sussman LS, Allore HG, Gill TM. Restricting symptoms in the last year of life: a prospective cohort study. JAMA Intern Med. 2013;173(16):1534–40.
Quinn KL, Shurrab M, Gitau K, Kavalieratos D, Isenberg SR, Stall NM, Stukel TA, Goldman R, Horn D, Cram P, et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic noncancer illness: a systematic review and meta-analysis. JAMA. 2020;324(14):1439–50.
Knaul FM, Farmer PE, Krakauer EL, De Lima L, Bhadelia A, Jiang Kwete X, Arreola-Ornelas H, Gomez-Dantes O, Rodriguez NM, Alleyne GAO, et al. Alleviating the access abyss in palliative care and pain relief-an imperative of universal health coverage: the Lancet Commission report. Lancet. 2018;391(10128):1391–454.
Luckett T, Phillips J, Agar M, Virdun C, Green A, Davidson PM. Elements of effective palliative care models: a rapid review. BMC Health Serv Res. 2014;14:136.
Gómez Batiste X, Connor S: Building integrated palliative care programs and services. WHO Collaborating Centre Public Health Palliative Care Programmes; 2017. [https://www.fnh.cl/publicaciones/BuildingIntegratedPalliativeCareProgramsandServices.pdf.] Accessed 24 Apr 2022.
Quill TE, Abernethy AP. Generalist plus specialist palliative care–creating a more sustainable model. N Engl J Med. 2013;368(13):1173–5.
Davidson P, Halcomb E, Hickman L, Phillips J, Graham B. Beyond the rhetoric: what do we mean by a “model of care”? Aust J Adv Nurs. 2006;23(3):47–55.
Palliative Care Australia: National Palliative Care Standards. 5th edition.; 2018. [https://palliativecare.org.au/wp-content/uploads/dlm_uploads/2018/11/PalliativeCare-National-Standards-2018_Nov-web.pdf.] Accessed 24 Apr 2022.
Training.cochrane.org. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.3. [https://training.cochrane.org/handbook.] Accessed 16 Nov 2022.
Cochrane Handbook for Systematic Reviews of Interventions. Table 8.2.b: Reaching an overall risk-of-bias judgement for a specific outcome. [https://training.cochrane.org/handbook/current/chapter-08.] Accessed 16 Nov 2022
Ahronheim JC, Morrison RS, Morris J, Baskin S, Meier DE. Palliative care in advanced dementia: a randomized controlled trial and descriptive analysis. J Palliat Med. 2000;3(3):265–73.
Bekelman DB, Allen LA, McBryde CF, Hattler B, Fairclough DL, Havranek EP, Turvey C, Meek PM. Effect of a collaborative care intervention vs usual care on health status of patients with chronic heart failure: the CASA Randomized clinical trial. JAMA Intern Med. 2018;178(4):511–9.
Bassi I, Guerrieri A, Carpano M, Gardini A, Prediletto I, Polastri M, Curtis JR, Nava S. Feasibility and efficacy of a multidisciplinary palliative approach in patients with advanced interstitial lung disease. A pilot randomised controlled trial Pulmonology. 2021;27:27.
Gade G, Venohr I, Conner D, McGrady K, Beane J, Richardson RH, Williams MP, Liberson M, Blum M, Della Penna R. Impact of an inpatient palliative care team: a randomized control trial. J Palliat Med. 2008;11(2):180–90.
Hanson LC, Kistler CE, Lavin K, Gabriel SL, Ernecoff NC, Lin FC, Sachs GA, Mitchell SL. Triggered palliative care for late-stage dementia: a pilot randomized trial. J Pain Symptom Manage. 2019;57(1):10–9.
Helgeson SA, Burnside RC, Robinson MT, Mack RC, Ball CT, Guru PK, Moss JE: Early versus usual palliative care consultation in the intensive care unit. Am J Hosp Palliat Care® 2022:104990912211157.
Janssen K, Rosielle D, Wang Q, Kim HJ. The impact of palliative care on quality of life, anxiety, and depression in idiopathic pulmonary fibrosis: a randomized controlled pilot study. Respir Res. 2020;21(1):2.
Kluger BM, Miyasaki J, Katz M, Galifianakis N, Hall K, Pantilat S, Khan R, Friedman C, Cernik W, Goto Y, et al. Comparison of integrated outpatient palliative care with standard care in patients with parkinson disease and related disorders: a randomized clinical trial. JAMA Neurol. 2020;77(5):551–60.
O’Donnell AE, Schaefer KG, Stevenson LW, DeVoe K, Walsh K, Mehra MR, Desai AS. Social Worker-Aided Palliative Care Intervention in High-risk Patients With Heart Failure (SWAP-HF): a pilot randomized clinical trial. JAMA Cardiol. 2018;3(6):516–9.
O’Riordan DL, Rathfon MA, Joseph DM, Hawgood J, Rabow MW, Dracup KA, De Marco T, Pantilat SZ. Feasibility of implementing a palliative care intervention for people with heart failure: learnings from a pilot randomized clinical trial. J Palliat Med. 2019;22(12):1583–8.
Rogers JG, Patel CB, Mentz RJ, Granger BB, Steinhauser KE, Fiuzat M, Adams PA, Speck A, Johnson KS, Krishnamoorthy A, et al. Palliative care in heart failure: the PAL-HF randomized, controlled clinical Trial. J Am Coll Cardiol. 2017;70(3):331–41.
Schunk M, Le L, Syunyaeva Z, Haberland B, Tänzler S, Mansmann U, Schwarzkopf L, Seidl H, Streitwieser S, Hofmann M, et al. Effectiveness of a specialised breathlessness service for patients with advanced disease in Germany: a pragmatic fast-track randomised controlled trial (BreathEase). Eur Respir J. 2021;58(2):2002139.
Sidebottom AC, Jorgenson A, Richards H, Kirven J, Sillah A. Inpatient palliative care for patients with acute heart failure: outcomes from a randomized trial. J Palliat Med. 2015;18(2):134–42.
Aiken LS, Butner J, Lockhart CA, Volk-Craft BE, Hamilton G, Williams FG. Outcome evaluation of a randomized trial of the PhoenixCare intervention: program of case management and coordinated care for the seriously chronically ill. J Palliat Med. 2006;9(1):111–26.
Bajwah S, Ross JR, Wells AU, Mohammed K, Oyebode C, Birring SS, Patel AS, Koffman J, Higginson IJ, Riley J. Palliative care for patients with advanced fibrotic lung disease: a randomised controlled phase II and feasibility trial of a community case conference intervention. Thorax. 2015;70(9):830–9.
Brännström M, Boman K. Effects of person-centred and integrated chronic heart failure and palliative home care. PREFER: a randomized controlled study. Eur J Heart Fail. 2014;16(10):1142–51.
Brumley R, Enguidanos S, Jamison P, Seitz R, Morgenstern N, Saito S, McIlwane J, Hillary K, Gonzalez J. Increased satisfaction with care and lower costs: results of a randomized trial of in-home palliative care. J Am Geriatr Soc. 2007;55(7):993–1000.
Eggers C, Dano R, Schill J, Fink GR, Timmermann L, Voltz R, Golla H, Lorenzl S. Access to end-of life parkinson’s disease patients through patient-centered integrated healthcare. Front Neurol. 2018;9:627.
Evans CJ, Bone AE, Yi D, Gao W, Morgan M, Taherzadeh S, Maddocks M, Wright J, Lindsay F, Bruni C, et al. Community-based short-term integrated palliative and supportive care reduces symptom distress for older people with chronic noncancer conditions compared with usual care: A randomised controlled single-blind mixed method trial. Int J Nurs Stud. 2021;120: 103978.
Farquhar MC, Higginson IJ, Fagan P, Booth S. The feasibility of a single-blinded fast-track pragmatic randomised controlled trial of a complex intervention for breathlessness in advanced disease. BMC Palliat Care. 2009;8:9.
Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, Todd CJ, Booth S. The clinical and cost effectiveness of a Breathlessness Intervention Service for patients with advanced non-malignant disease and their informal carers: mixed findings of a mixed method randomised controlled trial. Trials. 2016;17:185.
Gao W, Wilson R, Hepgul N, Yi D, Evans C, Bajwah S, Crosby V, Wilcock A, Lindsay F, Byrne A, et al. Effect of short-term integrated palliative care on patient-reported outcomes among patients severely affected with long-term neurological conditions: a randomized clinical trial. JAMA Netw Open. 2020;3(8): e2015061.
Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, McCrone P, Booth S, Jolley CJ, Moxham J. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med. 2014;2(12):979–87.
Janssens J-P, Weber C, Herrmann François R, Cantero C, Pessina A, Matis C, Merlet Viollet R, Boiche-Brouillard L, Stirnemann J, Pautex S. Can early introduction of palliative care limit intensive care, emergency and hospital admissions in patients with severe chronic obstructive pulmonary disease? A Pilot Randomized Study Respiration. 2019;97(5):406–15.
Scheerens C, Pype P, Van Cauwenberg J, Vanbutsele G, Eecloo K, Derom E, Van Belle S, Joos G, Deliens L, Chambaere K. Early integrated palliative home care and standard care for end-stage COPD (EPIC): a phase II Pilot RCT testing feasibility, acceptability, and effectiveness. J Pain Symptom Manage. 2020;59(2):206-224.e207.
Ng AYM, Wong FKY. Effects of a home-based palliative heart failure program on quality of life, symptom burden, satisfaction and caregiver burden: a randomized controlled trial. J Pain Symptom Manage. 2018;55(1):1–11.
Wong FK, Ng AY, Lee PH, Lam PT, Ng JS, Ng NH, Sham MM. Effects of a transitional palliative care model on patients with end-stage heart failure: a randomised controlled trial. Heart. 2016;102(14):1100–8.
Higginson IJ, Evans CJ, Grande G, Preston N, Morgan M, McCrone P, Lewis P, Fayers P, Harding R, Hotopf M, et al. Evaluating complex interventions in end of life care: the MORECare statement on good practice generated by a synthesis of transparent expert consultations and systematic reviews. BMC Med. 2013;11:111.
Oriani A, Dunleavy L, Sharples P, Perez Algorta G, Preston NJ. Are the MORECare guidelines on reporting of attrition in palliative care research populations appropriate? a systematic review and meta-analysis of randomised controlled trials. BMC Palliat Care. 2020;19(1):6.
Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ. 2002;325(7365):652–4.
Hui D, Glitza I, Chisholm G, Yennu S, Bruera E. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer. 2013;119(5):1098–105.
Beernaert K, Smets T, Cohen J, Verhofstede R, Costantini M, Eecloo K, Van Den Noortgate N, Deliens L. Improving comfort around dying in elderly people: a cluster randomised controlled trial. Lancet. 2017;390(10090):125–34.
Buckingham S, Kendall M, Ferguson S, MacNee W, Sheikh A, White P, Worth A, Boyd K, Murray SA, Pinnock H. HELPing older people with very severe chronic obstructive pulmonary disease (HELP-COPD): mixed-method feasibility pilot randomised controlled trial of a novel intervention. NPJ Prim Care Respir Med. 2015;25:15020.
Chapman DG, Toseland RW. Effectiveness of advanced illness care teams for nursing home residents with dementia. Soc Work. 2007;52(4):321–9.
Di Pollina L, Guessous I, Petoud V, Combescure C, Buchs B, Schaller P, Kossovsky M, Gaspoz JM. Integrated care at home reduces unnecessary hospitalizations of community-dwelling frail older adults: a prospective controlled trial. BMC Geriatr. 2017;17(1):53.
Engelhardt JB, McClive-Reed KP, Toseland RW, Smith TL, Larson DG, Tobin DR. Effects of a program for coordinated care of advanced illness on patients, surrogates, and healthcare costs: a randomized trial. Am J Manag Care. 2006;12(2):93–100.
Fischer SM, Cervantes L, Fink RM, Kutner JS. Apoyo con Cariño: a pilot randomized controlled trial of a patient navigator intervention to improve palliative care outcomes for Latinos with serious illness. J Pain Symptom Manage. 2015;49(4):657–65.
Grande GE, Todd CJ, Barclay SI, Farquhar MC. Does hospital at home for palliative care facilitate death at home? Randomised controlled trial BMJ. 1999;319(7223):1472–5.
Hanks GW, Robbins M, Sharp D, Forbes K, Done K, Peters TJ, Morgan H, Sykes J, Baxter K, Corfe F, et al. The imPaCT study: a randomised controlled trial to evaluate a hospital palliative care team. Br J Cancer. 2002;87(7):733–9.
Higginson IJ, McCrone P, Hart SR, Burman R, Silber E, Edmonds PM. Is short-term palliative care cost-effective in multiple sclerosis? a randomized phase II trial. J Pain Symptom Manage. 2009;38(6):816–26.
Hughes SL, Cummings J, Weaver F, Manheim L, Braun B, Conrad K. A randomized trial of the cost effectiveness of VA hospital-based home care for the terminally ill. Health Serv Res. 1992;26(6):801–17.
Kimbell B, Murray SA, Byrne H, Baird A, Hayes PC, MacGilchrist A, Finucane A, Brookes Young P, O’Carroll RE, Weir CJ, et al. Palliative care for people with advanced liver disease: a feasibility trial of a supportive care liver nurse specialist. Palliat Med. 2018;32(5):919–29.
Kinley J, Hockley J, Stone L, Dewey M, Hansford P, Stewart R, McCrone P, Begum A, Sykes N. The provision of care for residents dying in U.K. nursing care homes. Age Ageing. 2014;43(3):375–9.
Kinley J, Stone L, Dewey M, Levy J, Stewart R, McCrone P, Sykes N, Hansford P, Begum A, Hockley J. The effect of using high facilitation when implementing the Gold standards framework in care homes programme: a cluster randomised controlled trial. Palliat Med. 2014;28(9):1099–109.
Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial. J Am Geriatr Soc. 2004;52(5):675–84.
Pantilat SZ, O’Riordan DL, Dibble SL, Landefeld CS. Hospital-based palliative medicine consultation: a randomized controlled trial. Arch Intern Med. 2010;170(22):2038–40.
Rabow MW, Dibble SL, Pantilat SZ, McPhee SJ. The comprehensive care team: a controlled trial of outpatient palliative medicine consultation. Arch Intern Med. 2004;164(1):83–91.
Taube E, Kristensson J, Midlöv P, Jakobsson U. The use of case management for community-dwelling older people: the effects on loneliness, symptoms of depression and life satisfaction in a randomised controlled trial. Scand J Caring Sci. 2018;32(2):889–901.
Temkin-Greener H, Mukamel DB, Ladd H, Ladwig S, Caprio TV, Norton SA, Quill TE, Olsan TH, Cai X. Impact of nursing home palliative care teams on end-of-life outcomes: a randomized controlled trial. Med Care. 2018;56(1):11–8.
Thoonsen B, Groot M, Engels Y, Prins J, Verhagen S, Galesloot C, van Weel C, Vissers K. Early identification of and proactive palliative care for patients in general practice, incentive and methods of a randomized controlled trial. BMC Fam Pract. 2011;12:123.
Thoonsen B, Vissers K, Verhagen S, Prins J, Bor H, van Weel C, Groot M, Engels Y. Training general practitioners in early identification and anticipatory palliative care planning: a randomized controlled trial. BMC Fam Pract. 2015;16:126.
Zimmer JG, Groth-Juncker A, McCusker J. Effects of a physician-led home care team on terminal care. J Am Geriatr Soc. 1984;32(4):288–92.
Zimmer JG, Groth-Juncker A, McCusker J. A randomized controlled study of a home health care team. Am J Public Health. 1985;75(2):134–41.
Amado J, Vasquez R, Huari R, Rimache L, Lizonde R. Impact of applying palliative care on symptoms and survival of patients with advanced chronic disease admitted to the emergency department. Indian J. 2020;26(3):332–7.
Bakitas MA, Dionne-Odom JN, Ejem DB, Wells R, Azuero A, Stockdill ML, Keebler K, Sockwell E, Tims S, Engler S, et al. Effect of an early palliative care telehealth intervention vs usual care on patients with heart failure: the ENABLE CHF-PC randomized clinical trial. JAMA Intern Med. 2020;180(9):1203–13.
Boogaard JA, de Vet HC, van Soest-Poortvliet MC, Anema JR, Achterberg WP, van der Steen JT. Effects of two feedback interventions on end-of-life outcomes in nursing home residents with dementia: a cluster-randomized controlled three-armed trial. Palliat Med. 2018;32(3):693–702.
Cox CE, Riley IL, Ashana DC, Haines K, Olsen MK, Gu J, Pratt EH, Al-Hegelan M, Harrison RW, Naglee C et al: Improving racial disparities in unmet palliative care needs among intensive care unit family members with a needs-targeted app intervention: The ICUconnect randomized clinical trial. Contemp Clin Trials 2021, 103 (no pagination).
Duenk R, Verhagen S, Bronkhorst E, Van Mierlo P, Broeders M, Collard S, Dekhuijzen R, Vissers K, Heijdra Y, Engels Y. Proactive palliative care for patients with COPD (PROLONG): a pragmatic cluster controlled trial. Palliat Med. 2017;28(12):2795–806.
Forbat L, Liu W-M, Koerner J, Lam L, Samara J, Chapman M, Johnston N. Reducing time in acute hospitals: a stepped-wedge randomised control trial of a specialist palliative care intervention in residential care homes. Palliat Med. 2020;34(5):571–9.
Kavalieratos D, Harinstein ME, Rose B, Lowers J, Hoydich ZP, Bekelman DB, Allen LA, Rollman BL, Ernecoff NC, Moreines LT, et al. Primary palliative care for heart failure provided within ambulatory cardiology: a randomized pilot trial. Heart Lung. 2022;56:125–32.
Koffman J, Yorganci E, Murtagh F, Yi D, Gao W, Barclay S, Pickles A, Higginson I, Johnson H, Wilson R, et al. The AMBER care bundle for hospital inpatients with uncertain recovery nearing the end of life: the improvecare feasibility cluster RCT. Health Technol Assess. 2019;23(55):1–150.
Koffman J, Yorganci E, Yi D, Gao W, Murtagh F, Pickles A, Barclay S, Johnson H, Wilson R, Sampson L, et al. Managing uncertain recovery for patients nearing the end of life in hospital: a mixed-methods feasibility cluster randomised controlled trial of the AMBER care bundle. Trials [Electronic Resource]. 2019;20(1):506.
Ma J, Chi S, Buettner B, Pollard K, Muir M, Kolekar C, Al-Hammadi N, Chen L, Kollef M, Dans M. Early palliative care consultation in the medical ICU: a cluster randomized crossover trial. Crit Care Med. 2019;47(12):1707–15.
Lindell KO, Klein S, Veatch MJ, Gibson KF, Kass D, Nouraie S, Rosenzweig MQ. Nurse-led palliative care clinical trial improves knowledge and preparedness in caregivers of patients with idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2021;18(11):1811–21.
Liu WM, Koerner J, Lam L, Johnston N, Samara J, Chapman M, Forbat L. Improved quality of death and dying in care homes: a palliative care stepped wedge randomized control trial in australia. J Am Geriatr Soc. 2020;68(2):305–12.
Schmucker AM, Flannery M, Cho J, Goldfeld KS, Grudzen C, The EI, Blaum C, Bischof J, Ouchi K, Elie M-C, et al. Data from emergency medicine palliative care access (EMPallA): a randomized controlled trial comparing the effectiveness of specialty outpatient versus telephonic palliative care of older adults with advanced illness presenting to the emergency department. BMC emerg. 2021;21(1):1–11.
Shinall MC, Karlekar M, Martin S, Gatto CL, Misra S, Chung CY, Porayko MK, Scanga AE, Schneider NJ, Ely EW, et al. COMPASS: A pilot trial of an early palliative care intervention for patients with end-stage liver disease. J Pain Symptom Manage. 2019;58(4):614–614.
Solari A, Giordano A, Patti F, Grasso MG, Confalonieri P, Palmisano L, Ponzio M, Borreani C, Rosato R, Veronese S, et al. Randomized controlled trial of a home-based palliative approach for people with severe multiple sclerosis. Mult Scler. 2018;24(5):663–74.
Van den Block L, Honinx E, Pivodic L, Miranda R, Onwuteaka-Philipsen BD, van Hout H, Pasman HRW, Oosterveld-Vlug M, Ten Koppel M, Piers R, et al. Evaluation of a palliative care program for nursing homes in 7 Countries: the PACE cluster-randomized clinical trial. JAMA Intern Med. 2020;180(2):233–42.
Arnold SV, Spertus JA, Lei Y, Allen KB, Chhatriwalla AK, Leon MB, Smith CR, Reynolds MR, Webb JG, Svensson LG, et al. Use of the Kansas City Cardiomyopathy questionnaire for monitoring health status in patients with aortic stenosis. Circ Heart Fail. 2013;6(1):61–7.
Hauser K, Walsh D. Visual analogue scales and assessment of quality of life in cancer. J Support Oncol. 2008;6(6):277–82.
Downar J, Goldman R, Pinto R, Englesakis M, Adhikari NK. The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. CMAJ. 2017;189(13):E484–93.
Thomas K, Watson M, Armstrong J, Wilson and the GSF team. : The Gold Standards Framework Proactive Identification Guidance (PIG) (7th edition). The Gold Standards Framework Centre In End of Life Care. Edited by CIC; 2022. [https://goldstandardsframework.org.uk/cd-content/uploads/files/PIG/Proactive%20Identification%20Guidance%20v7%20(2022).pdf.] Accessed 17 Nov 2022.
O’Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, Rogers JG, Leier CV, Stevenson LW. Triage after hospitalization with advanced heart failure: the ESCAPE (Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness) risk model and discharge score. J Am Coll Cardiol. 2010;55(9):872–8.
Ryerson CJ, Vittinghoff E, Ley B, Lee JS, Mooney JJ, Jones KD, Elicker BM, Wolters PJ, Koth LL, King TE, et al. Predicting survival across chronic interstitial lung disease: the ILD-GAP Model. Chest. 2014;145(4):723–8.
Naqvi IH, Mahmood K, Ziaullaha S, Kashif SM, Sharif A. Better prognostic marker in ICU - APACHE II, SOFA or SAP II! Pak J Med Sci. 2016;32(5):1146–51.
McColl E: Best practice in symptom assessment: a review. Gut 2004, 53 Suppl 4(Suppl 4):iv49–54.
Herr KA, Garand L. Assessment and measurement of pain in older adults. Clin Geriatr Med. 2001;17(3):457–78, vi.
Aiyegbusi OL, Roydhouse J, Rivera SC, Kamudoni P, Schache P, Wilson R, Stephens R, Calvert M. Key considerations to reduce or address respondent burden in patient-reported outcome (PRO) data collection. Nat Commun. 2022;13(1):6026.
Kroenke K, Stump TE, Monahan PO. Agreement between older adult patient and caregiver proxy symptom reports. J Patient Rep Outcomes. 2022;6(1):50.
Evans CJ, Benalia H, Preston NJ, Grande G, Gysels M, Short V, Daveson BA, Bausewein C, Todd C, Higginson IJ, et al. The selection and use of outcome measures in palliative and end-of-life care research: the MORECare International Consensus Workshop. J Pain Symptom Manage. 2013;46(6):925–37.
Murtagh FE, Ramsenthaler C, Firth A, Groeneveld EI, Lovell N, Simon ST, Denzel J, Guo P, Bernhardt F, Schildmann E, et al. A brief, patient- and proxy-reported outcome measure in advanced illness: Validity, reliability and responsiveness of the Integrated Palliative care Outcome Scale (IPOS). Palliat Med. 2019;33(8):1045–57.
Wang TJ. Concept analysis of functional status. Int J Nurs Stud. 2004;41(4):457–62.
Habraken JM, van der Wal WM, Ter Riet G, Weersink EJ, Toben F, Bindels PJ. Health-related quality of life and functional status in end-stage COPD: a longitudinal study. Eur Respir J. 2011;37(2):280–8.
Jang H, Lee K, Kim S, Kim S. Unmet needs in palliative care for patients with common non-cancer diseases: a cross-sectional study. BMC Palliat Care. 2022;21(1):151.
Abernethy AP, Shelby-James T, Fazekas BS, Woods D, Currow DC. The Australia-modified Karnofsky Performance Status (AKPS) scale: a revised scale for contemporary palliative care clinical practice [ISRCTN81117481]. BMC Palliat Care. 2005;4:7.
Theofilou P. Quality of life: definition and measurement. Eur J Psychol. 2013;9(1):p150-162.
Dy SM, Pfoh ER, Salive ME, Boyd CM. Health-related quality of life and functional status quality indicators for older persons with multiple chronic conditions. J Am Geriatr Soc. 2013;61(12):2120–7.
Squiers L, Peinado S, Berkman N, Boudewyns V, McCormack L. The health literacy skills framework. J Health Commun. 2012;17(Suppl 3):30–54.
Lewis JM, DiGiacomo M, Currow DC, Davidson PM. Dying in the margins: understanding palliative care and socioeconomic deprivation in the developed world. J Pain Symptom Manage. 2011;42(1):105–18.
Matsuyama RKBWIK, Lyckholm L, Wilson-Genderson M, Smith TJ. Will patients want hospice or palliative care if they do not know what it is? J Hosp Palliat Nurs. 2011;13:p41-46.
Bausewein C, Daveson B, Benalia H, Simon ST, Higginson IJ. : Outcome Measurement in Palliative Care The Essentials. PRISMA. 2014. [https://www.eapcnet.eu/wp-content/uploads/2021/03/Outcome-Measurement-in-Palliative-Care-The-Essentials-.pdf.] Accessed 24 Apr 2022.
Bausewein C, Daveson BA, Currow DC, Downing J, Deliens L, Radbruch L, Defilippi K, Lopes Ferreira P, Costantini M, Harding R, et al. EAPC White Paper on outcome measurement in palliative care: improving practice, attaining outcomes and delivering quality services - recommendations from the European Association for Palliative Care (EAPC) task force on outcome measurement. Palliat Med. 2016;30(1):6–22.
Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9.
Ellis-Smith C, Higginson IJ, Daveson BA, Henson LA, Evans CJ. BuildCare: How can a measure improve assessment and management of symptoms and concerns for people with dementia in care homes? a mixed-methods feasibility and process evaluation of IPOS-Dem. PLoS ONE. 2018;13(7): e0200240.
Bradshaw A, Santarelli M, Mulderrig M, Khamis A, Sartain K, Boland JW, Bennett MI, Johnson M, Pearson M, Murtagh FEM. Implementing person-centred outcome measures in palliative care: an exploratory qualitative study using normalisation process theory to understand processes and context. Palliat Med. 2021;35(2):397–407.
Acknowledgements
We thank all the authors who participated in this research; Dr. Catherine J Evans for providing utmost support, Dr. Matthew Maddocks for checking the protocol, Ms. Elizabeth Alejandra Dominguez Palomerae and Ms. Rebeka Torlay for the support of an independent full paper review. This work was financially supported by JST SPRING. The author A.K. would like to take this opportunity to thank the ‘Interdisciplinary Frontier Next-Generation Researcher Programme of the Tokai Higher Education and Research System’.
Funding
The author A.K. is funded by JST SPRING (Grant Number JPMJSP2125) and C.J.E. by Health Education England/ National Institute of Health and Care Research (HEE/NIHR), Senior Clinical Lectureship (Grant Number ICA-SCL-2015–01-001). The views expressed in this publication are those of the authors and not necessarily those of the JST, the NHS, the NIHR or the Department of Health and Social Care. The funding agencies are not directly involved in the design of the study, collection, analysis, interpretation of data, or in writing the manuscript.
Author information
Authors and Affiliations
Contributions
Conceptualisation: C.J.E.; Methodology: A.K., C.J.E.; Data analysis and Writing: A.K.; Supervision: C.J.E.. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Kawashima, A., Evans, C.J. Needs-based triggers for timely referral to palliative care for older adults severely affected by noncancer conditions: a systematic review and narrative synthesis. BMC Palliat Care 22, 20 (2023). https://doi.org/10.1186/s12904-023-01131-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12904-023-01131-6